|
|
Biocompatible ABS CYCOLAC NOVODUR MAGNUM LUSTRAN
Model No.︰
MED ABS M203FC
Brand Name︰
medical abs resin
Country of Origin︰
China
Unit Price︰
-
Minimum Order︰
25 KG
Total 20 Related Items
Product Description
Medical grade ABS resin
ISO10993-5,-10,
FDA,
ISO22196
MEDICAL ABS INVENTORY LIST
| Type |
Color No. |
Mat No. |
Oriign |
Available |
| CYCOLAC HMG47MD |
1H1000
8H4D025
|
22018625
-55lbs/bag
|
Canada
USA
|
on request
|
| CYCOLAC HMG94MD |
1H1000 |
22023443 |
USA |
1,000 kg |
| MAGNUM 8391MED |
N/A |
N/A |
USA |
on request |
| |
|
|
|
|
| |
|
|
|
|
|
Antimicrobl ABS
LM915NB
|
NP
White
|
ISO22196
ISO10993-5,-10,-23
ASTM G21
FDA 21CFR 181.32
|
Korea |
on request |
| |
|
|
|
|
| |
|
|
|
|
| Lustran H950 |
901510 black |
HDT 105℃ in 1.8Mpa |
EU |
500 kg |
| LUSTRAN 348 |
000000 natural
012002 snow-white (50020904)
|
Meets FDA modified ISO 10993-1 requirements
Meets U.S. Pharmacopeia 23 Class VI test requirements.
APPLICATIONS:Components of intravenous (IV)systems,Surgical instruments Diagnostic test kits
|
USA |
2 box
(726kg/box)
3 box
|
| NOVODUR M203FC |
NR |
50022637 |
Germany |
20 kg |
| ELIX ABS M203FC |
000000 |
99004132 |
SPAIN |
1,400 kg |
|
**Notes: Our inventory list changes every month, Any compounding more color for medical ABS application, Please send e-mail to : x.g.chiang@gmail.com
|
| |
Payment Terms︰
T/T
Packing︰
25KG/BAG
Lead Time︰
30 DAYS
Product Image
 MAGNUM 8391MED ABS MAGNUM 8391MED ABS
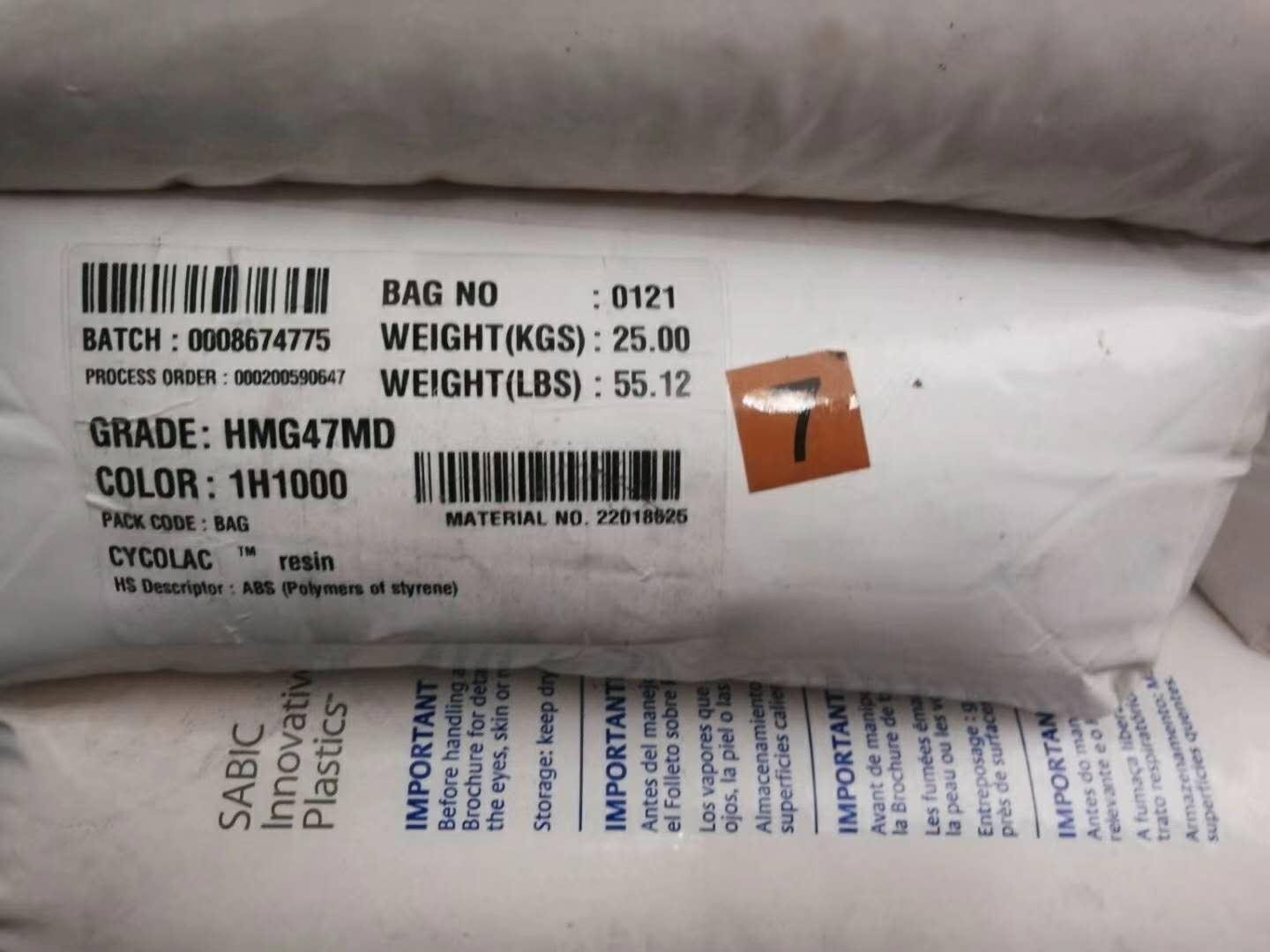 CYCOLAC HMG47MD CYCOLAC HMG47MD
 MEDICAL ABS MEDICAL ABS
 TAIRILAC AG16A1 TAIRILAC AG16A1
 TOYOLAC 950ME1 TOYOLAC 950ME1
 ELIX ABS M203FC ELIX ABS M203FC
 NOVODUR HD M203FC NOVODUR HD M203FC
 CYCOLAC HMG94MD CYCOLAC HMG94MD
 ABS Lustran 348 snow white 012002 ABS Lustran 348 snow white 012002
 LUSTRAN 348 LUSTRAN 348
|